Our understanding of our distant ancestors and their clans was formed in the nineteenth and twentieth centuries, and back then, there were no significant breakthroughs or discoveries. Skulls, jaws, and, more commonly, phalanges and teeth filled museums, prompting only subdued excitement in the specialized academic press. However, in the 2000s, everything changed with the emergence of a new science—paleogenetics. This discipline gave a voice to long-muted remains, unveiled previously undiscovered human species, and provided unparalleled insight into the lives of our ancestors, whose legacy had been preserved solely through bone fragments. Professor Alexander Markov recounts the incredible discoveries made in the last decade.
Three distinct human lineages—the Neanderthals, Denisovans, and Homo sapiens—once lived apart but eventually began to interact across the vast expanses of Eurasia. The Neanderthals, spreading eastward, encountered the Denisovans, possibly as early as 200,000 years ago. As Homo sapiens migrated out of Africa, they crossed paths with Neanderthals in the Near East. These initial encounters may have occurred some 130,000 or even 200,000 years ago. Over time, these interactions persisted, occurring in the Near East, South Asia, and Europe. In New Guinea, Homo sapiens coexisted with Denisovans, perhaps as recently as 30,000 to 15,000 years ago.
Regardless of the relationships between these tribes, which possessed different biological traits, appearances, and cultures, two things are certain: the Neanderthals and Denisovans became extinct shortly after the arrival of Homo sapiens. The Neanderthals disappeared, according to recent data, around 40,000 years ago. The Denisovans likely persisted for a bit longer but eventually vanished as well. However, the interactions among the Neanderthals, Denisovans, and Homo sapiens left a legacy. Not all of these encounters were strictly business—many turned out to be romantic. Nevertheless, as we now understand, the offspring of these unions were not particularly numerous or genetically robust because, by the time of intermingling, the three lineages had lived separately for too long, about half a million years.

Neanderthal man (Homo neanderthalensis) talking to a sitting elderly woman, artwork/MAURICIO ANTON/ SCIENCE PHOTO LIBRARY
So, the suitors and brides probably regarded each other with surprise, if not disdain, sometimes with curiosity. Nonetheless, we inherited genetic material from these extinct human groups, and recently, we've even learned to identify it within our genomes. On average, each Eurasian individual carries about 2 per cent Neanderthal DNA, and some Asian populations also have a small fraction of Denisovan DNA. These mixtures are absent or minimal in African genomes, but there are other genetic contributions from unknown extinct African populations (that I have mentioned in the previous lecture). What did these genetic mixtures impart to us? Paleogeneticists and specialists in comparative genomics continue to grapple with these fascinating questions.
The Challenges of Love
Why didn't Homo sapiens, Neanderthals, and Denisovans merge, instead likely retaining their genetic identity and distinctiveness until the end, that is, until the extinction of Neanderthals and Denisovans? That very distinctiveness allows us to confidently say, ‘This is a Neanderthal, that's a Denisovan, and this one is definitely a Homo sapiens.’
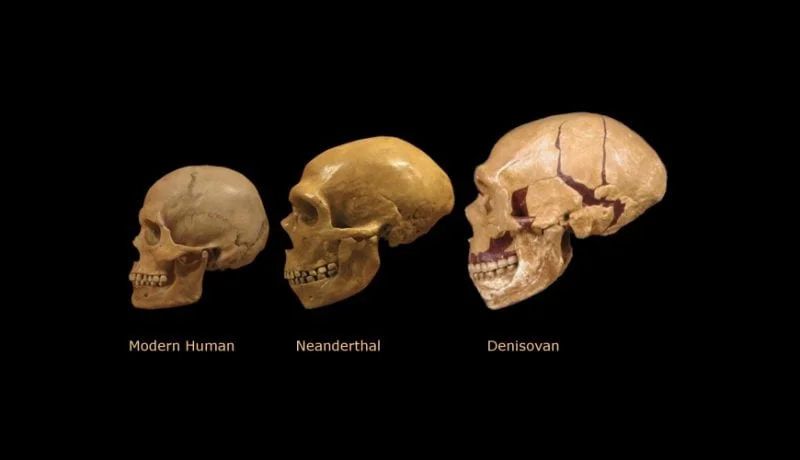
Skulls of Homo Sapiens, Neanderthals and Denisovans/from open access
Apparently, something still hindered them from merging, and this ‘something’ wasn't related to their willingness to interbreed because love, as they say, is blind. In all likelihood, the reason can be found in the hybrids' reduced viability or fertility.i
In their haste to utilize the new opportunities arising from the discovery of a high-quality Neanderthal genome, Svante Pääbo, David Reich, and their colleagues scanned the genomes of 1,004 modern individuals in search of Neanderthal genetic contributions. They developed a specialized computer program to assess the likelihood of any selected genomic segment with Neanderthal origins.
As a result, they compiled a registry of Neanderthal genetic mixtures in these 1,004 modern genomes. It was found that Neanderthal genes are unevenly distributed across the genomes of non-African Homo sapiens. In certain chromosomal regions of Asians and Europeans, Neanderthal contributions are as high as 62–64 per cent, while in others, they are entirely absent.

Svante Paabo with a skull/Getty images
The primary achievement was the scientists’ discovery of clear indications of partial reproductive incompatibility between Neanderthals and Homo sapiens. It appears that much of the Neanderthal genetic material introduced was actually harmful to Homo sapiens. Alien genes did not integrate well into the sapiens' genetic context and reduced the adaptability of hybrid offspring. In essence, oranges cannot grow from an apple tree, and over subsequent epochs, natural selection gradually phased out these unsuccessful genetic borrowings. Based on scientific estimates, right after hybridization, non-African sapiens initially carried between 3 to 6 per cent Neanderthal genes. As we recall, this percentage has since declined to about 2 per cent due to the effects of purifying selection.
What does this look like in practice? Firstly, among modern Europeans, Asians, and native Americans, the proportion of preserved Neanderthal fragments was highest in the least essential parts of the genome, those areas with the fewest functioning genes and regulatory sequences. Conversely, the most crucial genome regions have the least Neanderthal traces.
In some genome regions, Neanderthal borrowings have been entirely purged by selection, and this is especially true for our speech abilities. One such region, where modern humans have no Neanderthal mixture at all, includes the FOXP2 gene. Damage to this gene leads to speech problems. The protein encoded by the FOXP2 gene was the same in both the Neanderthals and us (but not the same as in chimpanzees and other primates). However, in the regulatory region of this gene in Homo sapiens, one mutation, which was not present in Neanderthals, became fixed. This affected something significant, judging by how carefully natural selection purged Neanderthal alleles from this part of the genome in non-African sapiens. Neanderthals did indeed possess speech, but how they spoke (or what they said) seems to have poorly matched the sapiens' notions of beauty. They probably grunted something incomprehensible to themselves.

Reconstruction of a group of Neanderthals/Max Planck Institute for Evolutionary Anthropology
Second, there were far fewer Neanderthal borrowings in the X chromosome than in the autosomes. This meant that males of mixed sapiens-Neanderthal origin apparently left, on average, less viable offspring compared to purebred competitors. Scientists have found that such individuals simply produced sperm less effectively. In response, natural selection subsequently purged Neanderthal admixtures from sapiens that negatively impacted sperm production.
This result starkly contrasts with the complete absence of any signs of genetic incompatibility between modern human populations, even in those that diverged quite some time ago, such as the Europeans and Africans. We were essentially like Martians to Neanderthals and Denisovans. Love, of course, is often unkind, and while we can understand what may have driven ancient people in their quest for love, the consequences of this passion clearly did not please nature.
The Others within Us
By studying the genomes of the Neanderthals and Denisovans and comparing them with our own, we may eventually understand why these species became extinct while we are still here. However, we must first remember that they evolved and thrived for hundreds of thousands of years. Our entire civilization is but a moment compared to their history. They adapted to conditions and inhabited climate zones that no primate before them dared to contemplate. Therefore, they undoubtedly had strengths in their biology that allowed them to adapt to life in Eurasia's vast, varied, and ever-changing landscapes. Consequently, Homo sapiens who acquired part of their genetic material through interbreeding with Denisovans and Neanderthals might have inherited some of their adaptive properties. The search for the adaptive legacy of the Denisovans and Neanderthals is an intriguing research topic, and work on this began immediately after the publication of the draft Neanderthal and Denisovan nuclear genomes.

Reconstructions of different stages of human evolution/Photo by P. Plailly
For instance, as early as 2011, a study was conducted to investigate the genes responsible for combating viral infections and recognizing regenerated cells within the body. In scientific terms, these human ‘air defense systems’ are known as the major histocompatibility complex (MHC). In total, three MHC class I genes, namely HLA-A, HLA-B, and HLA-C, are responsible for defending against viruses in humans. They are clustered together on chromosome 6. MHC class I genes exhibit extreme polymorphism, meaning that each of them exists in the gene pool in hundreds of variants (alleles). This diversity is nature's response to the constant assault of viruses on our bodies.
The diversity of MHC genes may be further supported by sexual selection. Many vertebrates choose partners based on individual scent, which is heavily influenced by the MHC peptide set. Often, preference is given to scents different from one's own. So, when you're attracted to their scent, the decision is likely not yours. Nature cleverly favors rare MHC alleles to reduce the susceptibility of your offspring to illness.
Similarly, the selection pressure from viral disease epidemics plays a role in enhancing the variety of protective proteins. Viruses adapt to the most common variants of their host's protective proteins, acquire the ability to overcome this defense, and subsequently, carriers with uncommon variants gain an advantage. Simply put, nothing troubles the fortunate possessors of such proteins.
It makes sense to suggest that among the genes non-African sapiens inherited from the Neanderthals and Denisovans, specific MHC alleles might have been especially beneficial to our ancestors. This is because sapiens who migrated out of Africa were likely less adapted to local infections than the indigenous Eurasian populations. Therefore, such borrowing could have provided them with advantages.
To test this hypothesis, scientists compared the sets of alleles of the HLA-A, HLA-B, and HLA-C genes in a girl from the Denisova Cave and three Neanderthals from Croatia with the allele diversity of these same genes in modern humanity. Nearly all MHC class I alleles found in Neanderthals and the Denisovan girl are present in the gene pool of modern humanity, with many being exclusive to regions outside tropical and southern Africa. This supports the idea that these alleles were acquired by our ancestors from Neanderthals and Denisovans as their absence in indigenous Africans would be difficult to explain otherwise.
The original Krapina 3 skull as illustrated in Der diluviale Mensch von Krapina in Kroatien (1906), by Dragutin Gorjanović-Kramberger published 1906/Wikimedia Commons
Some MHC alleles borrowed by non-African sapiens from the Denisovans and Neanderthals turned out to be advantageous and favored by natural selection, evident from how frequently they occur.
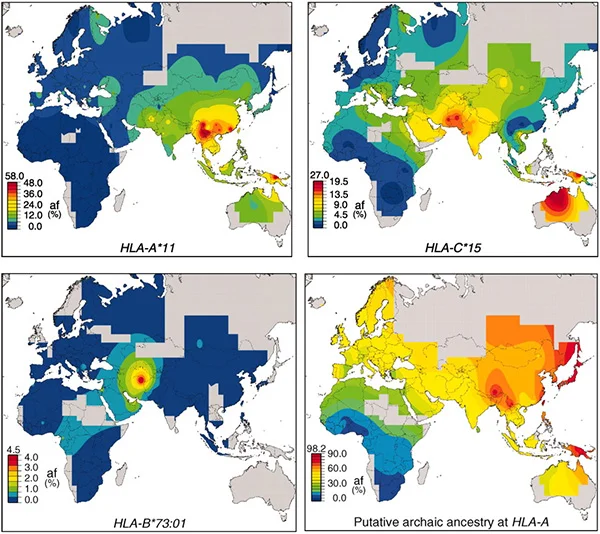
Maps showing the distribution of various Neanderthal and Denisovan genes among groups of modern humans/ from the article «The Shaping of Modern Human Immune Systems by Multiregional Admixture with Archaic Humans»//Science. 2011
For instance, the collective frequency of all presumed borrowed HLA-A alleles in certain parts of East Asia exceeds 60 per cent, and in mountainous regions of New Guinea, it approaches 100 per cent. One widely spread borrowed allele, HLA-A, effectively protects against the Epstein-Barr virus. As for the function of others, it remains unknown.
How the Denisovans Helped Us Conquer Mountains
In 2014, an international team of geneticists, led by Rasmus Nielsen from the Center for Theoretical and Evolutionary Genetics at the University of California, Berkeley, examined another notable instance of successful borrowing of alleles from our ancestors by modern humans, supported by natural selection. We've previously touched on this idea when discussing the Tibetan Denisovan. This pertains to the EPAS1 gene, which helps in adjusting to low oxygen levels, controlling blood pressure, and affecting the development and function of the heart muscle.
The study revealed that modern Tibetans have distinct alleles of this gene, which enhance the hemoglobin levels in erythrocytes while maintaining a consistent erythrocyte count. Carriers of these ‘Tibetan’ variants exhibit elevated hemoglobin levels regardless of their altitude of residence.

A Buddhist monk in Tibet/Wikimedia commons
Do these ‘high-altitude’ alleles occur in other populations? The Chinese, being the most closely related to Tibetans both territorially and historically, were examined. Scientists compared the DNA segment containing the EPAS1 gene in 40 Chinese and 40 Tibetans individuals respectively. The result was zero. In essence, it suggests poor adaptation for Chinese individuals in Tibet. Then, researchers examined data on this gene from global collections representing various races. Once again, the result was zero. No one else possessed it. Well, almost no one. There was one exception, warranting a big exclamation mark. The genome with a clear Tibetan signature belonged not to a modern human but to a Denisovan, specifically the girl from the Denisova Cave. Hence, modern Tibetans and ancient Denisovans shared a common adaptation to high-altitude hypoxia.

Portrait of a Denisova girl based on the morphology of the skull of Denisova 3, reconstructed by methyl. From the popular synopsis published in the journal Nature (Callaway, 2019) to the article by Gokhman et al., 2019 in the journal Cell/ILLUSTRATION DE MAAYAN HAREL
How did the Denisovan version of EPAS1 end up exclusively among the Tibetans? Scientists’ calculations suggest that the most likely scenario involves the Denisovans mating with the ancestors of both Chinese and Tibetans, resulting in mixed offspring. In one lineage branch, Denisovan EPAS1 alleles spread due to their adaptive advantage, while in another branch, these alleles were nearly lost because they weren’t necessary.
Initially, it was unclear why the Denisovans themselves required the high-altitude variants of EPAS1, given they lived only 700 meters above sea level. This question was clarified with the discovery of the Tibetan Denisovan, revealing that some Denisovans lived at high altitudes in the mountains.
How Neanderthals Turned Us into Early Risers and Gave Us Depression
Indeed, the Neanderthal existence was bleak, monotonous, and brief. Unlike sapiens, they lacked music and flashy attire (for a long time). They were oblivious to fashionable spots, with their communities remaining relatively small. With the same faces and conversations, everything was out in the open. In such an environment, anyone would likely succumb to depression. However, that's not the only reason. Eurasia experienced different light conditions compared to Africa, with shorter days in the winter and longer days in the summer.

Neanderthal man thought about life/Wikimedia commons
We are aware of Neanderthal alleles that marginally heighten the risk of depression in contemporary Europeans. Intriguingly, these Neanderthal genetic variations seem to impact the activity of genes associated with circadian rhythms. It's plausible that these Neanderthal alleles once conferred benefits to sapiens. Genes borrowed from Neanderthals may have aided newcomers in adjusting their ‘biological clocks’. However, with the progress of civilization and the advent of electric lighting, what proved advantageous for hunter-gatherers might have become harmful under new circumstances, potentially amplifying the likelihood of depression. It's known that this likelihood is partly correlated with lighting conditions and, presumably, the body's ability to adapt to them.

Zdenek Burian. Neanderthals/Wikimedia commons
Further research has revealed that sapiens and the ancient Eurasians (the Neanderthals and Denisovans) accumulated a significant number of genetic differences over time, especially in the genes related to regulating daily rhythms, during their separate existences on different continents. As sapiens migrated to high-latitude regions, they needed to adjust their biological clocks, and the Neanderthal genes acquired through hybridization assisted sapiens here. Interestingly, selectively favored Neanderthal genetic variants in sapiens increase the likelihood of the carrier being an ‘early bird’. This may be due to the fact that early birds (those with an early chronotype, to be precise) adapt more readily to seasonal changes in light conditions.
The Neanderthals Are to Blame for Covid
In 2020, during the height of the coronavirus pandemic, geneticists identified a segment of the human genome where nucleotide variations significantly influence the chances of contracting a severe form of Covid-19. This segment on the third chromosome includes six genes and occurs in several variants among modern humans. One of these variants, during the pandemic, increased the likelihood of hospitalization with severe Covid-19 by about 1.6 times. Paleogeneticists Svante Pääbo and Hugo Zeberg showed that this risk allele originates from the Neanderthals. The frequency of occurrence of this risk allele varies widely depending on the region. In Africa and East Asia, it is close to zero; in Europe, it is 8 per cent; and in South Asia, it is 30 per cent. The highest frequency is observed in Bangladesh at 38 per cent. Such large differences can only be explained by strong selection, which operates differently in different regions. It is logical to assume that the main factors in selection were some pathogens, such as disease agents. Perhaps the Neanderthal allele, which reduces resistance to the new coronavirus infection, was subjected to negative selection in China during previous epidemics caused by other coronaviruses, while in the Ganges delta, it was subjected to positive selection because it provided protection against other pathogens.
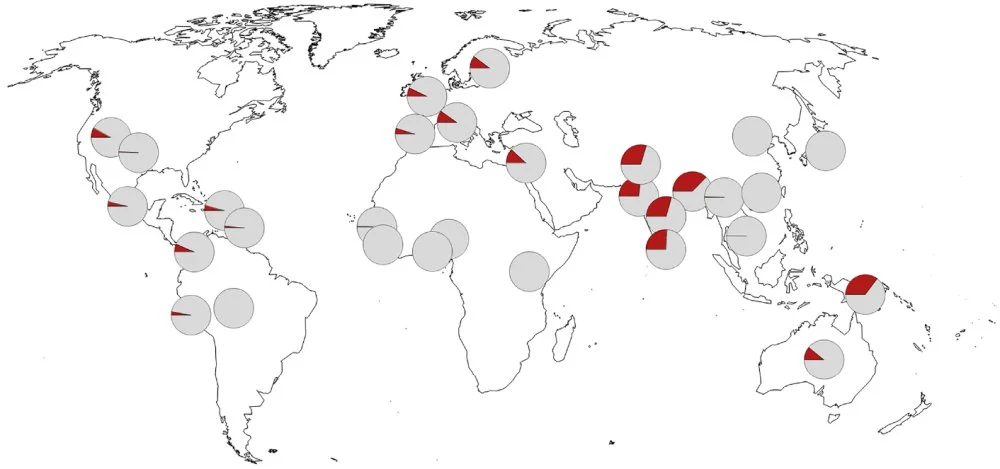
The frequency of occurrence of a Neanderthal genetic variant that increases the risk of severe COVID-19. In Africa and East Asia, the "risk allele" is practically absent, and the maximum frequency is observed in South Asia, especially in Bangladesh/Nature (2015)
In any case, the lives of ancient people continue within us, and paleogenetics may soon discover much more exciting information that will allow us to better understand not only them but also ourselves.
